Sperm-egg interaction in the palaemonid
shrimp,
Palaemonetes vulgaris
SEINEN CHOW1* AND
PAUL A. SANDIFER2
1National
Research Institute of Far Seas Fisheries, Shimizu 424-8633, Japan, 2South Carolina Department of Wildlife & Marine Resources,
Charleston, SC 29412, USA
*Corresponding author: Tel. +81-543-36-6045. Fax.
+81-543-35-9642.
E-mail: chow@enyo.affrc.go.jp.
@Sperm of the decapod
crustaceans are non-motile, having no flagella and hence no ability to swim
toward eggs. Ultrastructural studies have reported no
apparent acrosomal structure in sperm of caridean shrimp.1, 2 In contrast, sperm of other
decapod groups, such as Penaeidea,
Astacidea, Palinura, Anomura and Brachyura, possess acrosomal structures,1, 3-10 and drastic
structural changes of sperm morphology accompanied with acrosomal
reaction have been observed upon sperm-egg contact.1, 3, 4, 6
Sperm-egg interaction in caridean shrimp has been
observed only in the freshwater shrimp Macrobrachium
rosenbergii,11, 12 where the "thumb tack-" or "everted umbrella-" shaped sperm was observed to
penetrate the egg membrane with its spike. In this report, we present several
scanning electron microscope images of sperm-egg interaction in another palaemonid shrimp, the estuarine grass shrimp, Palaemonetes vulgaris.
Adult P. vulgaris were
obtained from Goose Creek in Charleston County, South Carolina, USA. Female and
male were held separately in 38 L aquaria at 25. An artificial photoperiod of 12 h light and dark was
kept, and faint light was maintained in the dark period for observation.
Females undergone the pre-spawning molt usually in the dark period, and they
were transferred to an aquarium containing males to allow mating. Mated females
were removed and isolated in another aquarium. Onset of spawning was observed
as the initial flowing of eggs into the abdominal brood chamber. In caridean shrimp, contact between eggs and non-motile sperm
apparently occurs as the eggs exit the ovipores and
enter the narrow, closed channel and brood chamber formed by the female pleopods and pleura.2,11 Samples of immediate
post-spawning (30 s to five min) eggs were collected from three females. Each
female was gently captured by a dip net and the eggs in its brood chamber were
removed by a pipette. The eggs were pre-fixed in 5% glutaraldehyde-0.07M sodium
cacodylate solution (pH 7.4) at room temperature,
kept for 4 to 12 h at 4
and post-fixed with 0.1% OsO4 in 0.1M phosphate buffer (pH 7.4) for
2 h at 4. The post-fixed eggs
were rinsed in 0.1M sodium cacodylate buffer (pH 7.4)
containing 7% sucrose for a minimum of one day, dehydrated by alcohol series,
and dried under critical point, followed by sputter-coating with gold for the
scanning electron microscope observation. The eggs were observed by a scanning
electron microscopy of a JEOL JSM 35C instrument at the Medical University of
South Carolina.
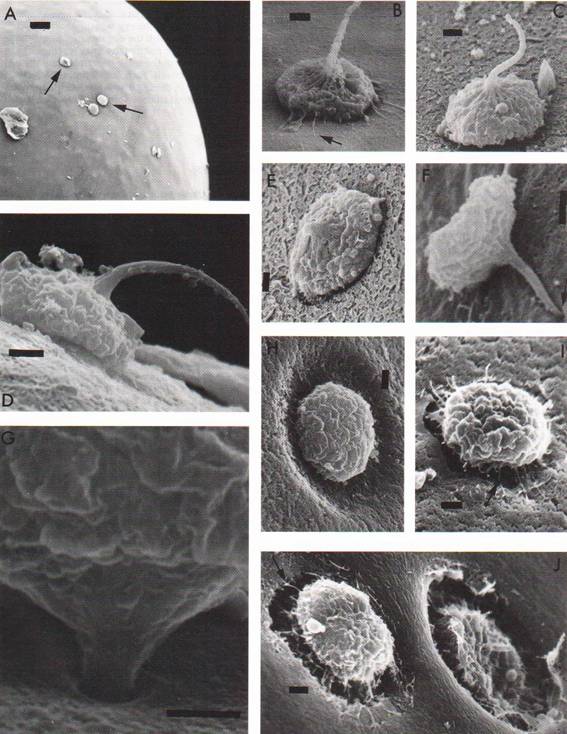
@Scanning microscopic images are shown in Fig. 1.
Multiple sperm cells were observed to attach to the egg outer investment coat
(OIC) of almost all eggs (Fig. 1, A). The sperm displayed the typical palaemonid morphology of a cupped base with a single spike
and were associated with the OIC in variable orientations (Fig. 1, B-F). Some sperm
cells were observed to penetrate the OIC (Fig. 1, G-J). Several
phases of sperm-egg interaction were sometimes observed even on a single egg.
The arrangement of our sequence of images of sperm-egg interaction for P. vulgaris
followed that reported for M. rosenbergii.12)
@In P. vulgaris, when the cupped base of the sperm attached to
the OIC, filamentous strands were observed to project from the sperm base,
usually in association with the OIC (Fig. 1, B, arrow). As reported for M. rosenbergii,
the sperm spike then apparently began to bend (Fig. 1, C, D) until the tip made
contact with the OIC (Fig. 1, E). The sperm base was partially detached from
the OIC and the tip of the spike pointed to the OIC (Fig. 1, F, arrow). The
sperm base was then entirely detached from the OIC and lifted as the spike
penetrated further into the OIC (Fig. 1, G). As the sperm continued to
penetrate the OIC, a depression (Fig. 1, H) was formed in the OIC. This
depression yielded a gaping hole in which the base of the sperm sank (Fig. 1,
I, J). Filamentous strands projected from the underside of the sperm base and
appeared to associate with the gaping hole (Fig. 1, I, J, arrows).
@The penetration of individual eggs by multiple sperm
(Fig. 1, J), suggests that polyspermy might be functional
or compensatory for ensuring fertilization in such non-motile sperm. Further,
as noted previously for M. rosenbergii, 12 there was no apparent acrosomal reaction in P.
vulgaris.
In external morphology, the sperm of caridean
and penaeid shrimp resemble each other. However,
structurally they show significant dissimilarities. In particular, the spike of the penaeid sperm is believed to be an elaborate acrosome complex,7 while the spike of caridean sperm consists of filaments.2
Furthermore, the spike of the penaeid shrimp sperm is
depolymerized upon sperm-egg contact,6 and
such reaction apparently does not occur in caridean
sperm.12 Upon contact with environmental water the OIC begins to
hydrate,13 and the sperm must enter the egg before entry is blocked
by this coat.
The
authors would like to thank Dr. Patricia S. Glas,
National Health and Environmental Effects Research Laboratory, Florida, USA,
for giving valuable comments on this manuscript.
REFERENCES
1. Brown GG. Ultrastructural
studies on crustacean spermatozoa and fertilization. PhD Thesis, University of
Miami, 1966.
2. Lynn JW, Clark WH Jr. The
fine structure of the mature sperm of the freshwater prawn, Macrobrachium rosenbergii. Biol. Bull. 1983; 164: 459-470.
3. Brown GG. Ultrastructural
studies of sperm morphology and sperm-egg interaction in the decapod Callinectes sapidus. J. Ultrastruct. Res. 1966; 14: 425-440.
4. Hinsch
GW. Penetration of the oocyte envelope by spermatozoa
in the spider crab. J. Ultrastruct. Res.. 1971; 35: 86-97.
5. Clark WH Jr., Talbot PT, Neal
RA, Mock CR, Salser BR. In vitro fertilization with non-motile spermatozoa of the brown
shrimp Penaeus aztecus. Mar. Biol. 1973; 22: 353-354.
6. Yudin
AI. Clark WH Jr., Kleve MG. An acrosome
reaction in natantian sperm. J. Exp. Zool. 1979; 210: 569-574.
7. Clark WH Jr., Kleve MG, Yudin AI. An acrosome reaction in natantian
sperm. J. Exp. Zool.
1981; 218: 279-291.
8. Talbot P, Chanmanon P.
Morphological features of the acrosome reaction of
lobster (Homarus)
sperm and the role of the reaction in generating forward sperm movement. J. Ultrastruct.
Res. 1980; 70: 275-286.
9. Talbot P, Summers RG. The
structure of sperm from Panulirus, the spiny lobster,
with special regard to the acrosome. J. Ultrastruct.
Res. 1978; 64: 341-351.
10. Tudge
CC, Jamieson BGM. Ultrastructure of the mature
spermatozoon of the coconut crab Birgus latro (Coenobitidae: Paguroidea: Decapoda). Mar. Biol. 1991; 108: 395-402.
11. Chow S, Ogasawara Y, Taki Y. Male reproductive system and fertilization of the palaemonid shrimp Macrobrachium rosenbergii. Bull.
Japan. Soc. Sci. Fish. 1982; 48: 177-183.
12. Lynn JW, Clark WHJr. A
morphological examination of sperm-egg interaction in the freshwater prawn, Macrobrachium rosenbergii. Biol. Bull. 1983; 164: 446-458.
13. Glas
PS, Courtney LA, Rayburn JR, Fisher WS. Embryonic coat of the grass shrimp Palaemonetes pugio. Biol. Bull. 1997; 192: 231-242.
Fig. 1 Scanning
electron microscope images of sperm-egg interaction. A, The egg surface,
showing several sperm cells attaching to the egg investment (arrows). Bar = 20 mm. B-E show sperm attaching to the egg investment by its base. Each bar
= 2 mm. B, Several filamentous
strands (arrow) are observed to associate with the egg investment. C, Spike
begins bending. D, Spike shows prominent bend toward the egg investment. E, The
point of the spike reaches the egg investment. F, The sperm base detaches from
the egg investment, and the point of the spike penetrates into the egg
investment (arrow). Bar = 2 mm. G-J show sperm
cells penetrating into the egg investment. Each bar = 2 mm. G, A spike is almost completely entered into the egg investment.
H, A depression is formed in the egg
investment as sperm penetration continues. I, A gaping hole is formed in the
egg investment and filamentous strands (arrow) are associated with the hole. J,
Two sperm are penetrating at adjacent positions.